Example 1
Hope for people with rare disease – new treatment approaches thanks partly to animal research
Timothy syndrome is a rare genetic disease caused by a mutation in a gene. This mutation prevents certain calcium channels closing correctly in cells. As a result, communication between nerve cells, heart muscles and other cell types is disrupted, causing heart problems, autism and epilepsy. However, new research approaches are bringing hope of an effective treatment.
Timothy syndrome research helpful for other neurological diseases too
Research into Timothy syndrome is not only furthering the development of a specific treatment for patients, but also providing valuable findings for other neurological diseases, as is illustrated by the work done by scientist Sergiu P. Pașca and his team at Stanford University. After 13 years of research, they developed a way of replicating the calcium channel malfunction using cerebral organoids, miniature brain structures obtained from human stem cells. Initial successes were achieved in the laboratory using simple cells, then in complex “mini brains” (organoids) in the laboratory and following transplantation of the organoids into the brains of rats.
From cell cultures to organoids
Pașca’s research started in 2009, when he grew neural cells from skin cells obtained from patients with Timothy syndrome. As a result, it was possible to visualise defects in the laboratory for the first time, even if only in a flat cell culture. To create more realistic models, the team developed three-dimensional cerebral organoids (“mini brains”) that simulate certain aspects of human brain development.
Initial success with new form of treatment
The team tested a new form of treatment that could partially reverse the gene mutation that causes Timothy syndrome. Experiments with organoids were successful, calcium channel activity normalised. These results show the treatment’s potential for patients.
Why animal testing was necessary
Before a treatment can be authorised for use in humans, it must have been shown to be safe and effective. Today, that still means testing it in a living organism. This is why the researchers transplanted human organoids into newborn rats and gave them the new treatment. The results were highly promising, since the same positive effects were observed as in the organoids. Animal testing served two main purposes: providing a way of investigating how human brain cells behave in a living brain and determining whether the treatment is effective and well tolerated in a complex organism.
The research combines modern alternative methods with essential animal testing for the purpose of developing a new treatment for Timothy syndrome and similar diseases, and of reducing animal testing to the necessary minimum.
Further information:
- About the rare disease Timothy syndrome
- About the research done by Sergiu Pasca
- Link to the article in Nature Communications

Figure: From stem cell culture to organoid transplantation into the living organism
Animal testing is often indispensable in biomedical research as a way of assessing the safety and efficacy of new treatments. It provides insights into the complex interactions that take place inside a living organism in a way that is not possible using alternative methods alone. Researchers are increasingly developing alternative methods such as organoids to further reduce the use of animals.
Example 2
Progress in diabetes treatment
Type 1 diabetes is an autoimmune disorder in which the immune system attacks the body, impeding the production of insulin, the substance that regulates blood sugar levels. People with the condition are reliant on synthetic insulin to stay healthy. Because type 1 diabetes primarily affects children and adolescents, there is a huge need for medicines that are simple to take.
Insulin you can swallow
Nowadays, patients mainly inject or, more rarely, inhale their insulin. Until now, it has been impossible to produce insulin in tablet form because the insulin is broken down in the digestive tract before it can even start to work. A research team from the University of Sydney has developed a novel form of insulin that is made up of tiny particles known as nanoparticles. These protect the insulin from the destructive effects of stomach acid so that the active substance can subsequently be absorbed into the body in the intestines.
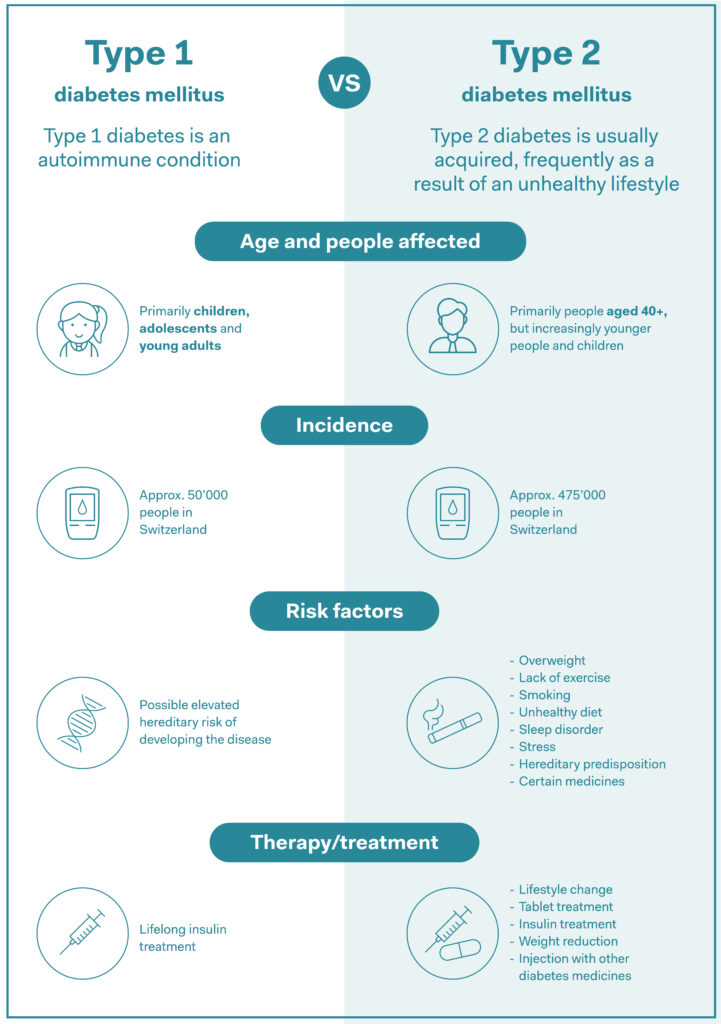
Source: AOK, own summary
Alternative methods play an important role in development
The new oral presentation was tested using alternative methods as well as animal testing. In the early stages of research, the scientists used in vitro models, particularly human intestinal tissue, to test how well the nanoparticles were absorbed. These tests showed a significant improvement in insulin absorption compared with conventional insulin. In addition, alternative model organisms such as Caenorhabditis elegans nematodes were used to obtain further information about the effects. However, further animal testing was still indispensable for a comprehensive assessment of the efficacy of the new insulin preparation. The research team investigated blood sugar reduction, prevention of hypoglycaemia and possible side effects in mice and baboons. These tests showed that the new presentation reliably reduced the animals’ blood sugar levels without any harmful side effects whatsoever. This is a major step forwards because up to now it was considered virtually impossible to make effective insulin in tablet form. Should further trials, including trials in humans, confirm the new dosage form’s efficacy, millions of diabetes patients worldwide could one day be spared the not unproblematic chore of injecting themselves with insulin.
A medicine that can prevent diabetes?
Moreover, a research team at Johns Hopkins University in Baltimore (USA) is working on a different approach to combating diabetes. They have developed a medicine that directly targets the cause of type 1 diabetes. The medicine in question is a monoclonal antibody, an active substance that specifically recognises and affects certain cells in the body. In this case, the antibody binds to cells known as beta cells in the pancreas. These produce insulin, but are attacked by the body’s own immune system cells in people with type 1 diabetes. The new medicine protects the beta cells against these attacks. Here again, animal testing was unavoidable for safety reasons. The efficacy of the new treatment was tested in mice with a high risk of developing type 1 diabetes. It was found that the animals who received the medicine did not develop the disease and lived longer than the untreated mice.
The as-yet indispensable tests with animals give rise to hope that the active substance may one day cure or even prevent diabetes. However, the medicine needs further development before it can be used in humans. Part of this involves developing a “humanised” version of the antibody, in other words an active substance that is specifically suitable for the human body.
Why alternative methods cannot (yet) replace animal testing
Both studies show that animal testing is still essential for progress in medicine in addition to alternative methods. It helps to determine the safety and efficacy of new treatments before they are used in the complex environment of the human biological system. In the case of both the insulin tablet and antibody-based medicine, animal testing yielded important findings that could pave the way for new forms of diabetes treatment.
Further information
- Nanotech opens door to future of insulin medication. The University of Sidney, 2 May 2024.
- Oral nanotherapeutic formulation of insulin with reduced episodes of hypoglycaemia, Nature, 2 January 2024.
- Experimental Type 1 Diabetes Drug Shelters Pancreas Cells from Immune System Attack, John Hopkins Medicine, 04/29/2024.
- Cell-Surface ZnT8 Antibody Prevents and Reverses Autoimmune Diabetes in Mice, Diabetes Volume 73, Issue 5, May 2024.
Animal testing is often indispensable in biomedical research as a way of assessing the safety and efficacy of new treatments. It provides insights into the complex interactions that take place inside a living organism in a way that is not possible using alternative methods alone. Researchers are increasingly developing alternative methods such as organoids to further reduce the use of animals.
Example 3
New antibiotic that combats antibiotic resistance discovered.
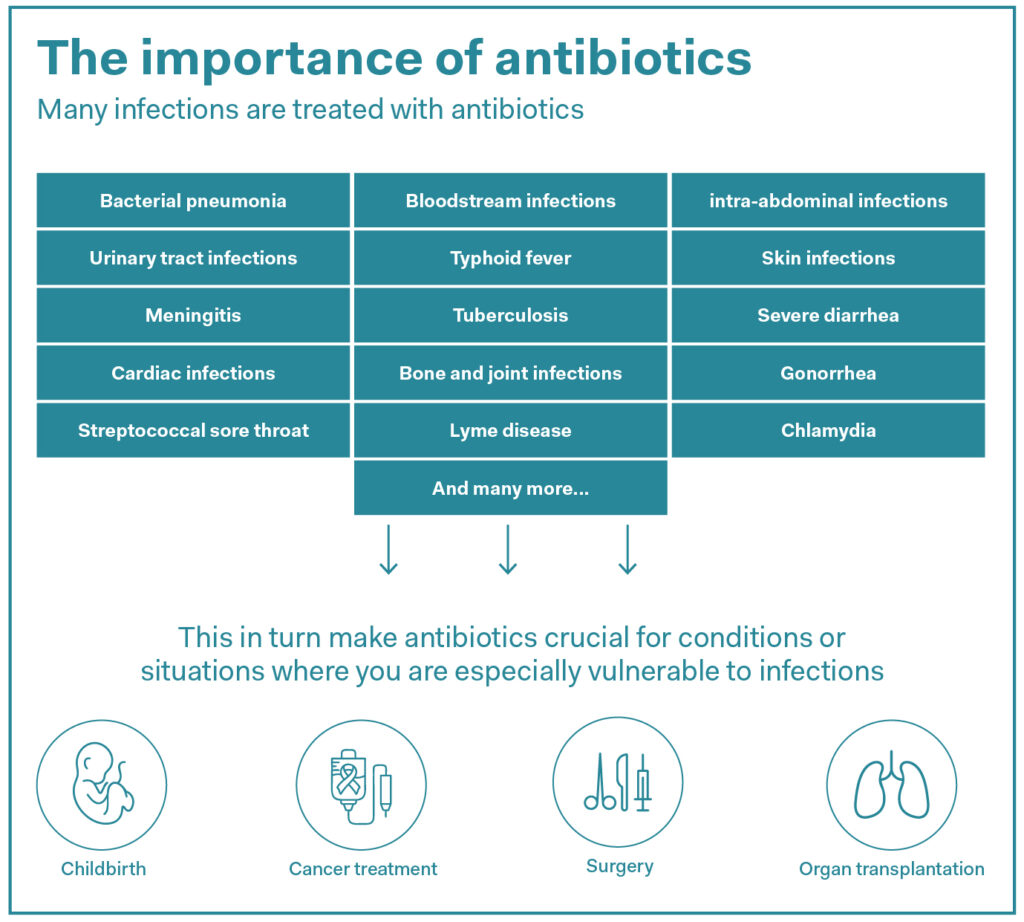
Antimicrobial or antibiotic resistance is the term used to describe the immunity of bacteria to antibiotics – crucial medicines that treat infections either by killing off bacteria or inhibiting their growth. Now widespread, antibiotic resistance arose as a result of excessive use of antibiotics in humans, animals, and plants and has become a global public health issue. According to current WHO estimates, around 4.71 million deaths worldwide were associated with bacterial antimicrobial resistance as of 2021.
Hope from the USA: targeted action that spares the gut
A team of researchers from the University of Illinois (USA) has now discovered a new antibiotic called Lolamicin. Under an approach that minimises animal use, the promising new active substance was first successfully tested against multidrug-resistant bacteria in cell culture (in vitro) and only then in mice. What makes Lolamicin special is its ability to kill dangerous bacteria while sparing healthy gut microbes. This is an innovation compared with conventional antibiotics, which often attack not only harmful, but also beneficial bacteria in the gut. This can disrupt the body’s equilibrium and have long-term consequences for health.

Source: WHO & OneHealthTrust
Proven high efficacy
Lolamicin inhibits a particular transport system in bacteria. This system is differently structured in harmful bacteria than in “good” gut bacteria. As a result, the medicine can specifically target and attack only the harmful bacteria. Lolamicin was given to mice during preclinical trials. The mice had either septicaemia or pneumonia caused by antibiotic-resistant bacteria. In an astonishing result, all the mice with septicaemia survived the trial, as did 70 percent of the mice with pneumonia. Not only that, treatment left the composition of the intestinal flora – the good bacteria in the digestive tract – virtually unchanged. Even 28 days after treatment, the animals were still completely in equilibrium.
Cell culture first, only then in mouse models
Lolamicin was not used in animal models until it had been tested on over 130 multidrug-resistant bacterial strains in cell culture. Cell culture is a step towards research without animal testing. Such in vitro models provide important information about the efficacy and toxicity of new active substances before they are tested in animal models. The tests with mice were a next important step because mice have a very similar microbiome (in other words, the community of bacteria in the gut) to humans. That means that findings from mouse studies can provide important pointers for subsequent trials in humans.
The discovery of Lolamicin could represent a genuine breakthrough in the fight against microbial resistance. It shows that it is possible to take specific action against dangerous bacteria without destroying the beneficial ones. However, the medicine has not yet been authorised for humans. Further studies of its safety and efficacy as well as the likelihood of it inducing resistance have still to be conducted. Nevertheless, the results give cause for hope. Perhaps there will soon be new antibiotics that we tolerate better, and which protect our health effectively despite the ongoing spread of resistance.
Further information:
- New antibiotic kills pathogenic bacteria, spares healthy gut microbes. By Diana Yates. University of Illinois Urbana-Champaign. May 29, 2024.
- Game-Changing Antibiotic Discovered That Spares ‘Good’ Bacteria. By Carly Cassella Health, 09 June 2024.
Animal testing is often indispensable in biomedical research as a way of assessing the safety and efficacy of new treatments. It provides insights into the complex interactions that take place inside a living organism in a way that is not possible using alternative methods alone. Researchers are increasingly developing alternative methods such as organoids to further reduce the use of animals.
Example 4
Tests in mice help develop novel potential treatment for a genetic skin disease
Congenital melanocytic naevus syndrome (CMN) is a rare genetic skin disease in which up to 80% of the patient’s skin is covered in large, itchy or even painful moles that can cause skin cancer. Unlike most moles, which do not develop until later, patients are born with the “nevi”, as they are called. Current treatment options are limited, and include complex surgery or laser treatment to remove the moles. However, removal is often incomplete, and the risk of cancer remains virtually unchanged. Medicinal treatment generally only alleviates the symptoms, such as itching pain or seizures.
Three-year-old helps major leap forward
Three-year-old Ada loves swimming and the sea, but a carefree visit to the beach is virtually impossible without particularly strict precautions. This is because she has CMN on 70% of her skin. The moles often cause her pain and itching. She donated skin cells to the Francis Crick Institute in London so they could be used in a research project investigating new treatment options.
The researchers used Ada’s cells to test an innovative new treatment process, the aim of which was to deactivate the mutated NRAS gene that causes the abnormal moles. Initial experiments in a Petri dish succeeded in suppressing the gene’s activity.
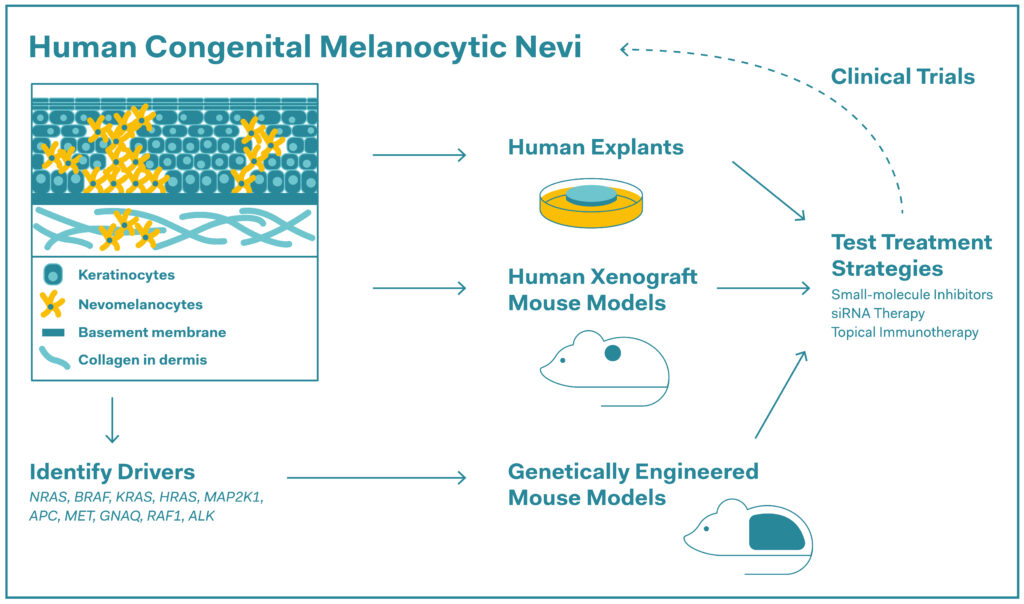
Preclinical research strategies to test repurposed therapies in congenital melanocytic nevi (CMN). Known drivers of CMN including genes with characterised mutations or fusions are listed and all discussed treatment strategies are included in the figure.
Source: Emerging Therapies for Congenital Melanocytic Nevi, 2025
Tests in mice fuel hopes
The next step was to test the potential therapy in animals – an essential step during which safety and efficacy are assessed in a living organism. The researchers injected the new medicine into mice with CMN. After just 48 hours, the effects were apparent. The mutated gene was being suppressed, and the abnormal skin changes started to recede.
Dr. Veronica Kinsler, Principal Group Leader and scientist at the Crick Institute and a member of the research project, is delighted, explaining that the disappearance of the moles and successful tests in mice represent milestones in CMN research. They offer hope for patients and their families. Although further testing is needed before the treatment can be trialled in humans, the studies in mice were nevertheless a necessary step in obtaining important data about the effect and risks of treatment.
Fresh hope for patients – and researchers
Ada’s parents also shared their views on the results. Knowing there could be a chance of their daughter’s CMN being reversed and a substantial reduction in her risk of developing skin cancer has blown their expectations out of the water. “It is mind-blowing to think that this treatment option could be available in only a matter of years.”
As well as being a leap forward in treatment, the project also highlights the strength of modern research. Thanks to a combination of laboratory cell-based models and animal testing, it is now possible to develop new treatments more specifically, faster and more safely. The study underscores the fact that although progress has been made on alternative methods, animal testing remains important in making the crucial final step to safe use in humans.
Sources:
- Researchers find potential of mole reversal therapy in rare condition. News an features; The Francis Crick Institute, 17 June 2024.
- RNA Therapy for Oncogenic NRAS-Driven Nevi Induces Apoptosis. Journal of Investigative Dermatology, Volum 145, Issue 1, P122-134, January 2025.
- Emerging Therapies for Congenital Melanocytic Nevi: Journal of Experimental Pathology, 2025 ;6 (1).
Animal testing is often indispensable in biomedical research as a way of assessing the safety and efficacy of new treatments. It provides insights into the complex interactions that take place inside a living organism in a way that is not possible using alternative methods alone. Researchers are increasingly developing alternative methods such as organoids to further reduce the use of animals.




